Evaluation of the state of charge and general technical condition of the battery.
The state of charge of the battery can be assessed subjectively or objectively, i.e.. based on measurements. Subjective assessment consists in observing the quality of work of car receivers, powered only from the battery and based on my own experience, that the battery is fully charged, or not, can it still be used, whether it needs to be recharged. The easiest way to infer the state of charge, judging correct operation of the starter. If its speed is slow or the starter motor lacks power to drive the engine, can be assumed, that the battery is discharged.
Observation of changes headlight level (e.g. dipped beam) with the engine off (battery powered only) and with the engine running (power supply from the generator) it also allows for an approximate assessment of the battery charge level. If the headlights both in the first, both light up the same - the battery is definitely well charged. When the engine is turned on, they glow much brighter, should be assumed, that the battery is discharged. This assessment is not fully reliable. The decrease in voltage at the terminals of the receivers used for the test may result not only from the decrease in the amount of electric charge in the battery as a result of discharge, but may be due to. its damage (e.g. internal short circuit), poor contact quality (connections) in the electric circuit, especially between the terminals and the conductors, which can increase the transition resistance, and thus the voltage drop at the connection point.
How to read the density of the electrolyte on the scale of the hydrometer: 1 - correct reading, 2 - incorrect reading.
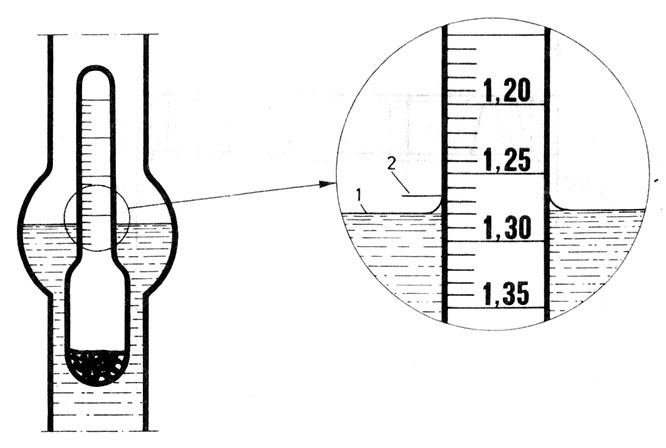
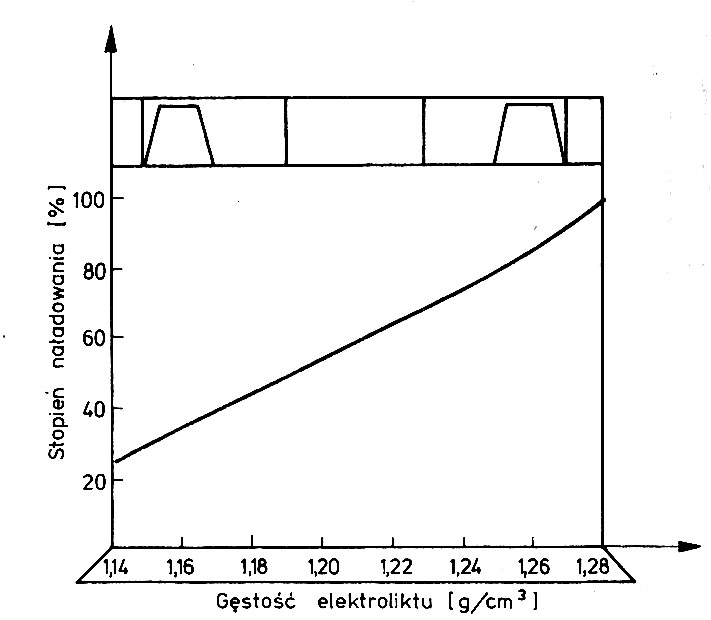
The most reliable result of assessing the degree of charge gives electrolyte density measurement or the voltage at the battery cell terminals. The density of the electrolyte in car batteries is measured using an acid meter. It consists of a glass pipette tipped with a rubber bulb. A hydrometer is placed in the pipette. The end of the pipette is inserted through the hole into the cell (after removing the cork) and sucks up the electrolyte in the same amount with a pear, for the hydrometer to float freely in it. The density is read on the hydrometer scale at the surface of the electrolyte. The density read on the scale is converted to the hydrometer scaling temperature (reference), i.e.. +25°C. With sufficient accuracy for practical purposes, it can be assumed, that the average value of the temperature change coefficient of the electrolyte density is 0,0007 g/(Cm3 ·°C). In other words, when recalculated or actually changed by 15°C, the density changes by approx 0,01 g/cm3. Sulfuric acid density (electrolyte) decreases with increasing temperaturetury. Knowing the density value at the reference temperature, determine the battery charge level on the basis of a graph or table.
Due to the possibility of permanent damage, the battery should not be discharged more than 25%, i.e.. to a density lower than 1,14 into 1,15 g/cm3.
Chart of changes in the battery charge level depending on the density of the electrolyte for temperature + 25°C.
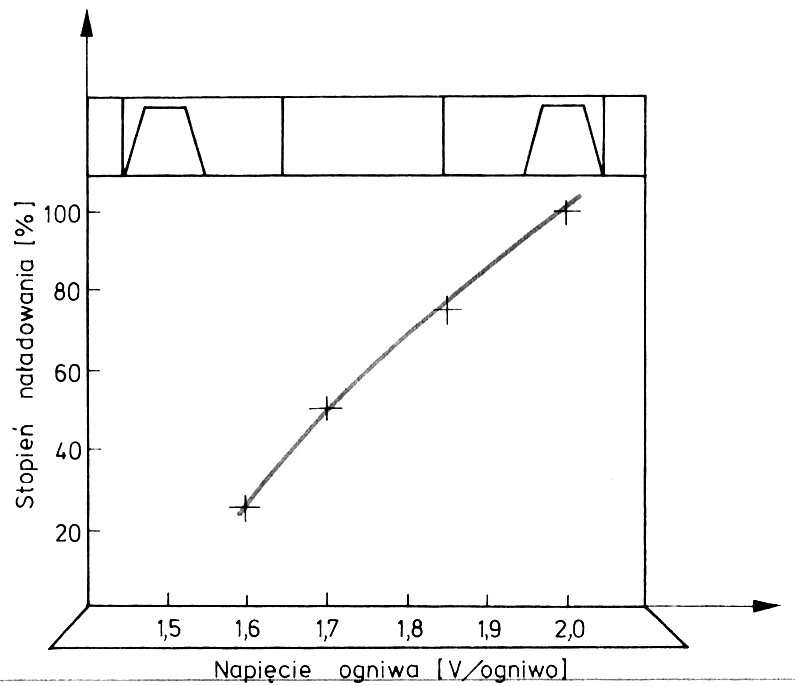
It can also be used to assess the state of charge of the battery voltage measurement at the ends of its links. The measurement is made with a loaded cell, i.e.. during current flow. It is accepted as a rule, that the current charging a battery cell with a capacity of twenty hours Q20 ≤ 100 A·h is 80 A, larger ones, i.e.. o Q20 > 100 A·h—150 A. By measuring the voltage, you can assess the state of charge of the batteries, whose links have their ends led outwards, thus exposed inter-cell connectors (the blocks of these accumulators are mostly made of ebonite). In currently produced non-disassembled batteries of small capacity (34, 45, 60 A h) terminals and connectors are hidden under the monolid and it is not possible to perform this measurement.
Chart for determining the battery charge level based on the measurement of the cell voltage under current load 80 A.
A special fork tester is used to measure the cell voltage under load. It is a voltmeter embedded in a holder, which is finished with a handle on one side, on the other, a replaceable resistor with two pointed rods (spades) pressed into the cell terminals during voltage measurement. The voltmeter is calibrated in voltage units (V), but apart from that, the scale is divided into differently colored areas indicating the percentage of the cell's charge.
Performing such a measurement at home is quite troublesome. First, you would need a sufficiently accurate meter (at least classes 2). Secondly, it is difficult to find a suitable receiver (e.g. resistor) with a power of approx 160 W and order resistance 25 mΩ. For example, if you want to use. from 12-volt car light bulbs 45 In, they should be connected in parallel 120!
With some approximation, you can assess the battery charge level, by measuring the voltage at its terminals under load. It is not an objective measurement. It is easiest to do it with the starter turned on (and with the high voltage cable removed from the distributor cap, that the engine will not start). The starter should run for a very short time, so as not to unnecessarily drain the battery. The voltmeter readings will correspond approximately to the state of the battery:
Ataku < 10,2 V - battery discharged,
10,2 V < Ataku < 11 V - battery charged in 50%,
Ataku > 11 V - battery charged.
Measuring the voltage at the ends of a cell or battery without load does not allow conclusions about the state of charge.
A nearly discharged battery may show the correct voltage, when it is loaded only with the current drawn by the voltmeter. This current is approx 1 mA, which practically does not count in the energy balance of the battery. The average current is consumed to power the ignition system itself 1500 times bigger!
This measurement can be helpful, when there is no voltage at the terminals or there is, but very small. No voltage while retaining through the plates (seen through the filler holes) a color indicating that they are charged (negative plates are grey-blue, positive - dark brown) indicates mechanical damage to internal connections (almost impossible to locate and remove in batteries with non-detachable blocks) or about battery leakage (which is impossible not to notice). In case of finding a reduced voltage at the battery terminals (with the correct electrolyte level) and if the color of the positive and negative plates seen through the filler hole is gray or off-white, can be suspected, that the battery has been sulphated and must be subjected to a desulphation charge first. However, it is worth pointing out here, that determining the color of the plates and drawing conclusions about the state of charge or sulphation on this basis is not easy and requires a lot of experience.
The assessment of the general technical condition, in addition to the discussed assessment of the degree of charge, also includes visual inspection of the battery. The condition of the housing is checked (block), mainly, is not broken, secure seating of the pole terminals (when the ends can be moved or rotated, this proves that they are detached from the bridges), overall cleanliness of the cover, especially near pole terminals and vent plugs.